Pfizer is seeking healthy unvaccinated individuals to participate in COVID clinical trials, telling those who avoided the first 6 shots that “now is the time to rethink your options for fighting COVID-19.”

“Now is the time to rethink your options for fighting COVID-19,” the invitation states.
Participants must be unvaccinated, at least 12 years old, and be generally healthy, with no history of myocarditis or pericarditis.
Also required to qualify for their clinical trial, participants must have been infected with COVID at least once, with the last positive test being at least 28 days prior.
“After having COVID-19, the natural immunity that occurs from infection may not provide enough protection against new variants of the virus,” the call to arms reads.
Pfizer added: “Your immunity also decreases over time. This means that you can still get sick from COVID-19 even if you have had it previously.”
The irony of Pfizer asking healthy unvaccinated individuals who avoided the first two shots and next four boosters to “rethink” their position, was not lost on many social media users.
Pfizer is asking unvaccinated as young as 12 years old to participate in a Covid vaccine study.
— 🅾️ Kat Kanada (@KatKanada_TM) September 12, 2023
🚩🚩🚩
To be eligible, you have to be
•generally healthy
•have no history of myocarditis or pericarditis. 🚨🚨🚨☠️
About this study ⬇️⬇️⬇️
———————-
This study will help us… pic.twitter.com/FcBSwor5nk
The invitation further states that “Your participation in this clinical trial could reduce your risk of getting COVID-19 again.”
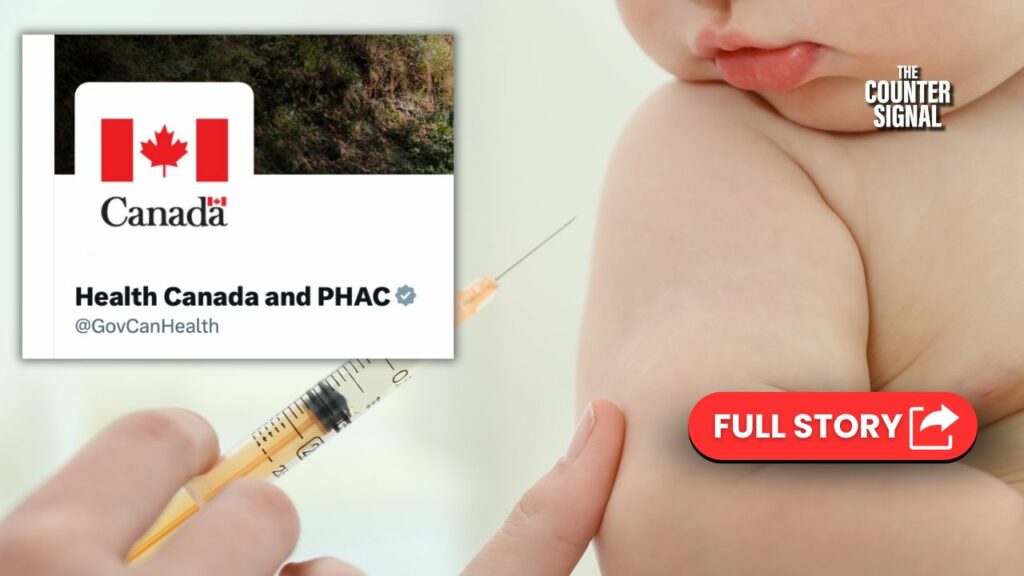
Health Canada approves “updated” vaccine
On Tuesday, Health Canada approved another COVID vaccine, called Spikevax XBB.1.5, and urged all Canadians over 6 months of age to get one unless they’ve been infected or gotten vaccinated in the past six months.
Health Canada just approved a new Moderna COVID vaccine for the XBB.1.5 variant for 6 month olds, kids, and teens, based on a study involving only 50 individuals:
— Ryan O'Connor (@rpoconnor) September 12, 2023
– 22% of whom were 65 or over
– 0% were under 21 years old
– 0% were monitored for adverse events after May 2023 pic.twitter.com/lgjESYFNFo
“Based on the totality of the information, the benefit-risk profile for a dose of Spikevax XBB.1.5 is considered favourable in individuals 6 months of age and older,” their update reads.
For some Canadians, this COVID booster would be their seventh shot in about 28 months.
Dr. Tam further said there is no evidence to suggest the new variants cause increased severity of disease.